what is an example of a ketone Ketones chemistry iupac
Hey there! Today, I want to talk about a topic that many students struggle with in chemistry: distinguishing between aldehydes and ketones. The good news is that with a little bit of practice and understanding of basic principles, it’s not that difficult! Let’s dive in. First, let’s take a look at the basic structure of aldehydes and ketones. Both types of compounds contain a carbonyl group, which is a carbon atom double-bonded to an oxygen atom. In aldehydes, the carbonyl group is located at the end of a carbon chain, while in ketones, it is located within the chain. One way to differentiate between aldehydes and ketones is by their physical properties. Aldehydes tend to have stronger odors than ketones, particularly if they are small molecules with fewer than six carbon atoms. This is because the carbonyl group in aldehydes is more exposed and therefore more easily able to interact with our senses of taste and smell. For example, formaldehyde, which is a simple aldehyde with just one carbon atom, has a very pungent odor. Another way to distinguish between aldehydes and ketones is by their reactivity. Aldehydes are more reactive than ketones because the carbonyl group in aldehydes is more polarized, making it more susceptible to attack by nucleophiles (substances that donate an electron pair). This reactivity can be seen in reactions such as the Tollens’ test and the Fehling’s test, which are used to distinguish aldehydes from ketones in the laboratory. So, now that we know the basics of distinguishing between aldehydes and ketones, how can we apply this knowledge in practice? One useful tool is to look at the functional groups present in the molecule and use the IUPAC naming system to identify the specific type of compound. For example, if the molecule contains a carbonyl group on the end of a carbon chain, it will be named as an aldehyde. If the carbonyl group is located within the chain, it will be named as a ketone. In conclusion, while distinguishing between aldehydes and ketones may seem daunting at first, with practice and a little bit of understanding of basic principles, it becomes relatively simple. By looking at the physical properties and reactivity of the compounds, as well as using the IUPAC naming system, we can easily identify the specific type of molecule that we are working with.
If you are searching about Henry’s Home: Ketone Bodies you’ve came to the right page. We have 5 Pics about Henry’s Home: Ketone Bodies like Nomenclature of Aldehydes & Ketones - Chemistry LibreTexts, Learning Chemistry Easily: June 2015 and also How to differentiate whether the compound is Aldehyde or Ketone Plz. Here you go:
Henry’s Home: Ketone Bodies
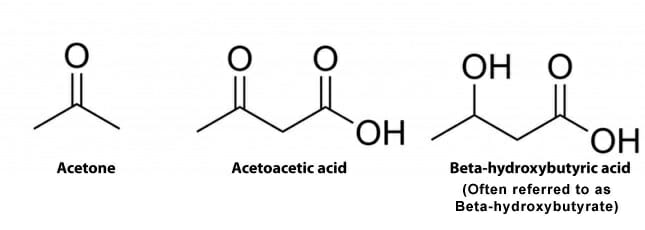 henryli3117.blogspot.comketone bodies ketosis keto body state definition acetoacetate three glucose middle
henryli3117.blogspot.comketone bodies ketosis keto body state definition acetoacetate three glucose middle
Nomenclature Of Aldehydes & Ketones - Chemistry LibreTexts
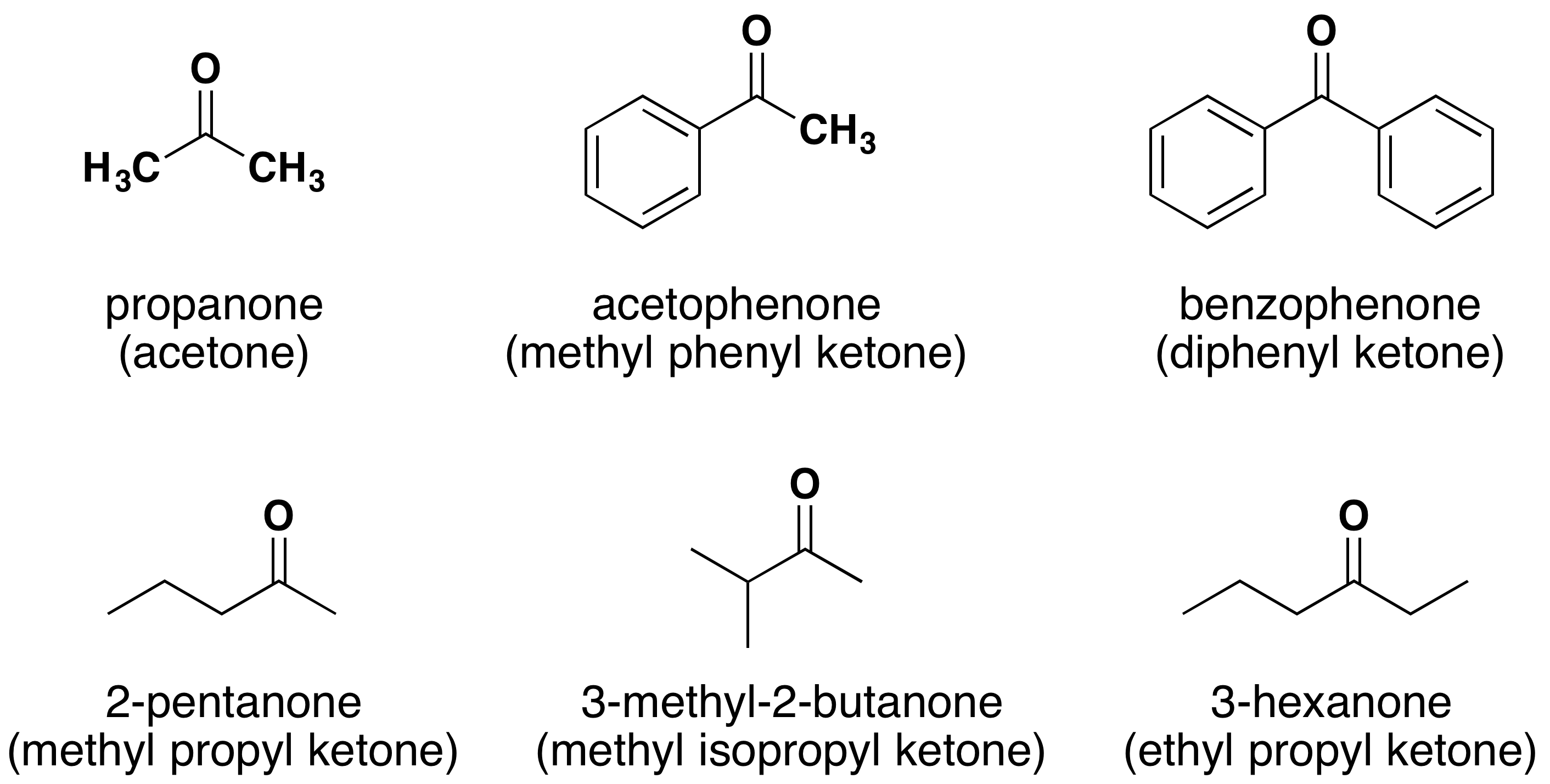 chem.libretexts.orgketones aldehydes nomenclature names common chemistry libretexts ketone naming iupac name organic carbonyl compounds molecule chem system acid
chem.libretexts.orgketones aldehydes nomenclature names common chemistry libretexts ketone naming iupac name organic carbonyl compounds molecule chem system acid
Learning Chemistry Easily: June 2015
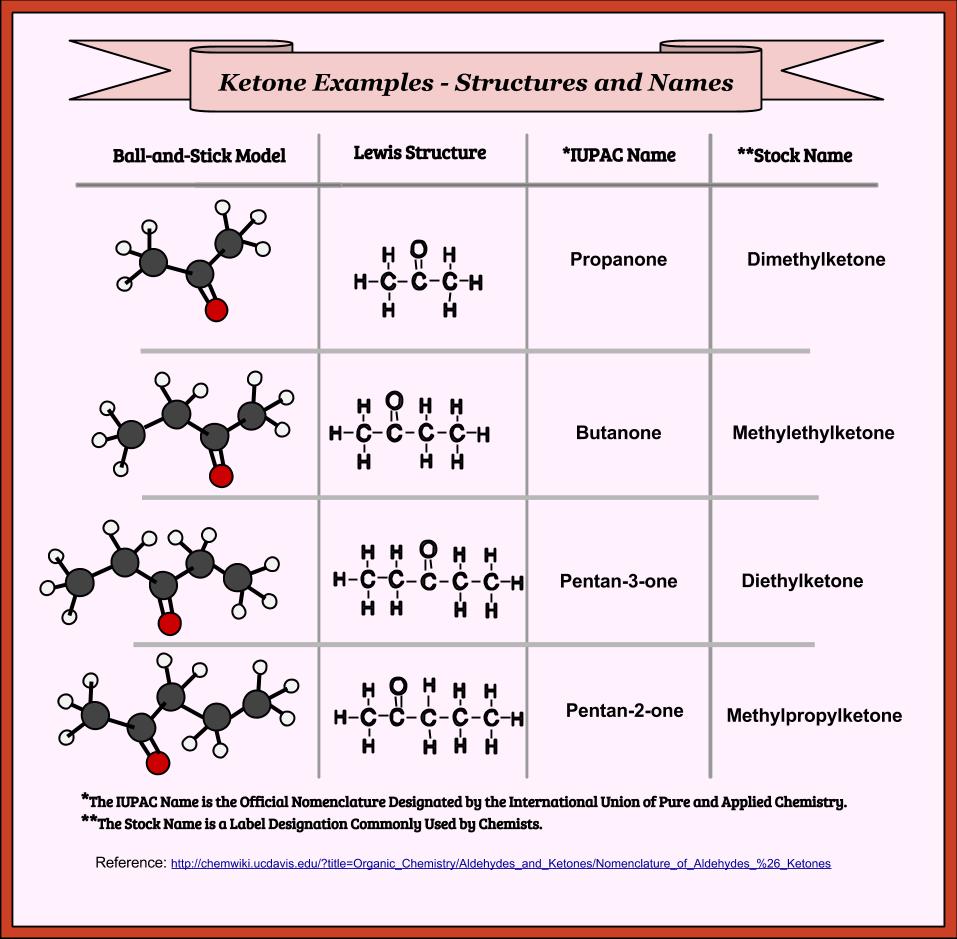 learningchemistryeasily.blogspot.comketones chemistry iupac
learningchemistryeasily.blogspot.comketones chemistry iupac
What Are Ketones? - Perfect Keto
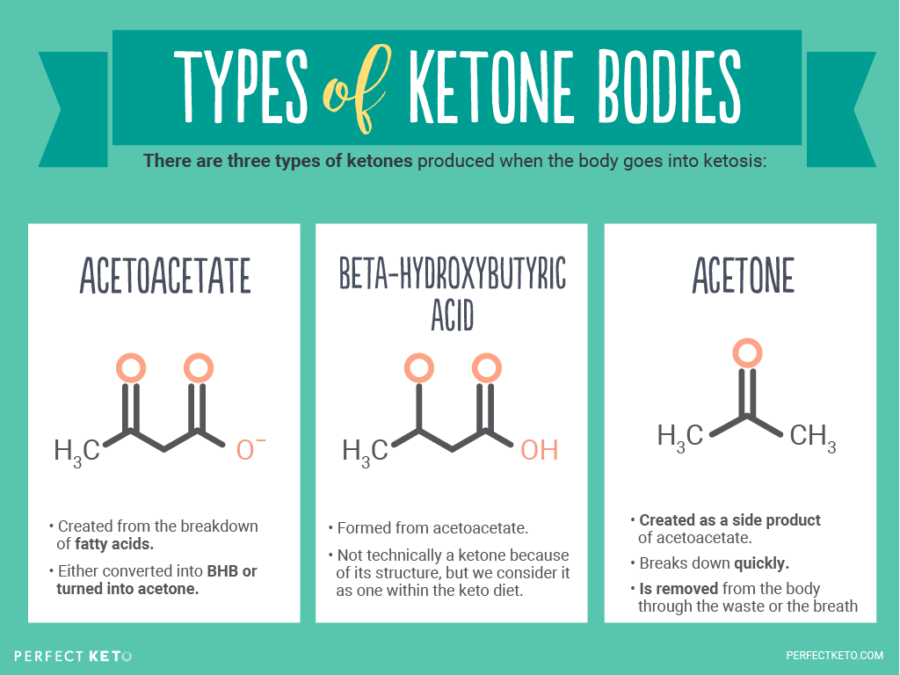 perfectketo.comketones ketone acetone bhb ketogenic acetoacetate hydroxybutyrate urine metabolism breath hydroxybutyric salts ketosis ketoacidosis perfectketo different fatty tissues nutritional exogenous
perfectketo.comketones ketone acetone bhb ketogenic acetoacetate hydroxybutyrate urine metabolism breath hydroxybutyric salts ketosis ketoacidosis perfectketo different fatty tissues nutritional exogenous
How To Differentiate Whether The Compound Is Aldehyde Or Ketone Plz
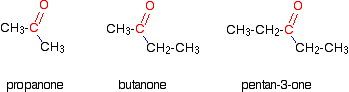 www.meritnation.comketone ketones example carbonyl group aldehyde compound differentiate whether explain plz atom hydrogen notice attached never propanone
www.meritnation.comketone ketones example carbonyl group aldehyde compound differentiate whether explain plz atom hydrogen notice attached never propanone
Nomenclature of aldehydes & ketones. How to differentiate whether the compound is aldehyde or ketone plz. Ketone ketones example carbonyl group aldehyde compound differentiate whether explain plz atom hydrogen notice attached never propanone